


Module 2: Chill
This week you’ll learn the role of ice and fat in creating a creamy ice cream!
Goal 1: Creamy
The first goal you’ll explore is how to make an ice cream, creamy. There’s a lot more to it than just adding cream. It all starts with changes of matter.
Changes of matter
Matter is anything that takes up space and can be weighed.
There are three main states that matter can exist in. They are:

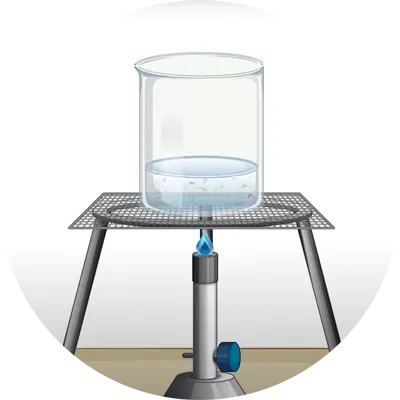
Now we know a change of matter is when one state of matter changes into another state. Like when ice melts into water, or when water heats up and turns into steam.
In ice cream, it looks something like this:
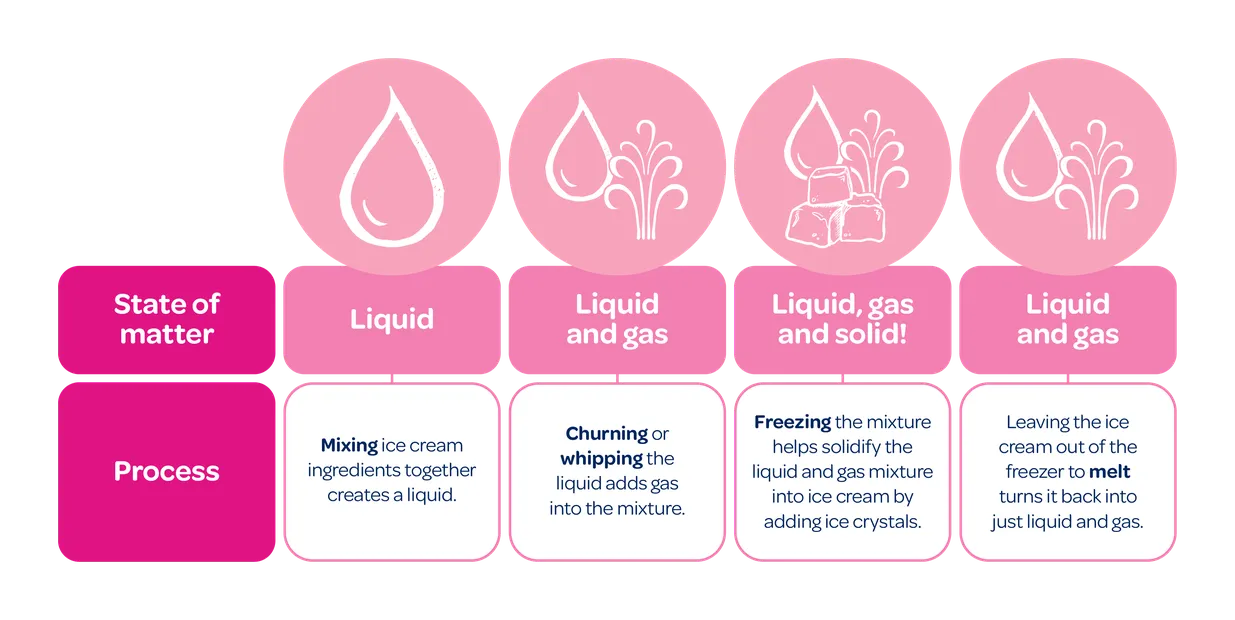
Why does it matter?
The ingredients in ice cream are all “matter”. When you make ice cream, the ingredients experience lots of different changes. And how they change, has a big effect on the creaminess of the ice cream.
Ice ice crystals
One of the changes that impacts creaminess is freezing – where a liquid changes into a solid when it’s cooled.
Cream and milk both have water in them. And when ice cream is frozen, the water molecules clump together to form ice crystals.
- If the ice crystals are big and unevenly spread, the ice cream will be icy and not very smooth.
- If the ice crystals are small and evenly spread, the ice cream will be smooth and creamy!
Activity 2.1: Ice crystal investigation
Investigate ice crystals and their impact on creaminess in extreme ice cream close-ups.
The clash of the crystals
It’s safe to say that big ice crystals are not welcome in creamy ice cream. Luckily, there are a few special STEM superpowers you can use to make sure they stay small and even.
Superpower 1: Quick, freeze!
When ice cream is frozen quickly, the ice crystals don’t have enough time to get big.
Superpower 2: Freezing point depression
Freezing point depression is when you lower water’s freezing temperature.
Water starts to freeze at a temperature of 0° Celsius. If you add something like salt or sugar to water, it makes it harder for ice crystals to form, and for the water to freeze.
This means the water molecules in ice cream won’t freeze until it the temperature gets even colder than 0°C. So, the ice cream will stay smooth and creamy in the freezer instead of icy!
Superpower 3: Fat vs frost
Cream and milk are both fats. In ice cream, they coat the ice crystals as they freeze. This prevents them from getting too big and uneven.
If the ice crystals stay small, the ice cream will be smooth and creamy instead of icy and crunchy. So, ice creams with more fat are usually creamier!
Activity 2.2: Ice cream in a bag
Test how fat and freezing makes ice cream creamy by creating an ice cream in a bag!

Update your sticker chart
Completed all your activities for this module? Don’t forget to put a sticker on your chart!
Reminder!
Start collecting trays and an electric beater. You’ll also need to freeze more ice and chill your cream and cold plates in the fridge overnight.
Module 2 checklist
Mīharo. Today you:
- Unpacked ice cream goal 1: creamy
- Learned about changes of matter
- Learned about ice crystals
- Explored how fat and freezing impacts ice crystals
- Created ice cream in a bag!